Beer-Lambert Law
A sample becomes more saturated with colour as the path length or concentration increases.
(Specifically, the relationship of the absorption of light to the properties of the material through which the light is travelling).
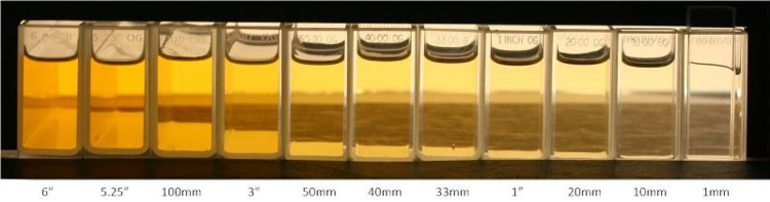
The diagram below shows Platinum Cobalt solution of 500 in various path length cells:
The cells used are, from left to right:
6", 5.25", 100mm, 3", 50mm, 40mm, 1", 20mm, 10mm, 1mm.
The exact same sample in a 6" cell appears vastly different to that in the 1mm cell.
As can be clearly seen, the saturation of the colour increases with the path length.
The same is true when the concentration of a sample increases, then so does the saturation of the colour.
This proves the importance when communicating colour:
it is essential to specify the path length of the cell.