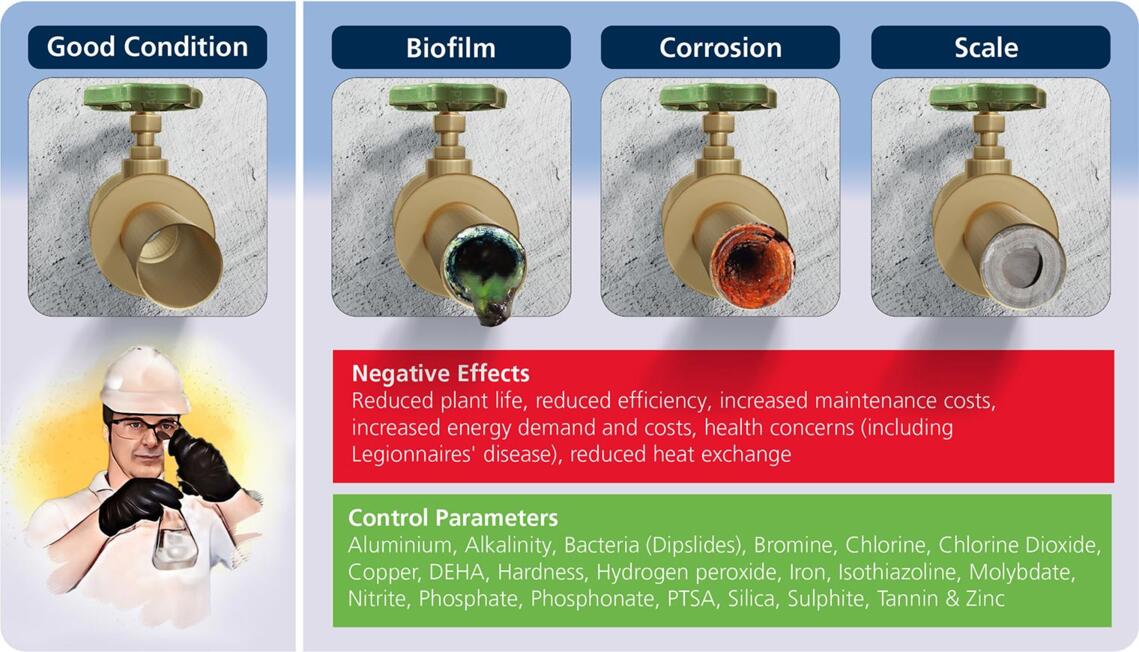
Biofilm
Microbes like other life forms have favourable conditions in which
they thrive. Unfortunately for people looking after cooling systems those, favourable conditions are just what we find in
evaporative cooling water systems, where microbes are particularly abundant due to the concentration of nutrients through the system 'cycling'. Due to the constantly wet surfaces in cooling water systems the abundant growth of microbes leads to the formation of biofilms. These biofilms, if left untreated, can lead to biofouling, resulting in reduced plant efficacy and potentially reducing plant life.
The microorganisms that inhabit cooling systems are typically common soil, aquatic and airborne microbes that enter the system either via make up water, process leaks or are scrubbed from the air and vary depending on the water source.
Their control depends on whether they are in a planktonic (free floating) or sessile (attached) form. The sessile form is responsible for biofilm formation. As noted, biofilms form on wetted surfaces, such as heat exchanger tubes, and the microorganisms that form them secrete polysaccharides when submerged, allowing them to form a gel-like network which prevents them from being removed by the normal flow of water and consequently, hinders the action of the action of a biocide, whether oxidising or non-oxidising. This is the reason the control of biofilms can require biocidal dosages many times higher than the control of planktonic species.
Once fouling has occurred in a system, even mechanical cleaning cannot remove all traces of the biofilm. Surfaces that have previously been fouled are more susceptible to colonisation than new surfaces, as residual biofilm materials promote growth and reduce lag time between fouling reappearing.
Some of the effects of biofilm formation:
- Biofilms act as insulation, where the performance of the heat exchanger deteriorates in correlation to the thickness of the biofilm.
- Biofilms can promote corrosion known as Microbial Influenced Corrosion (MIC), whereby the microbes act as catalysts for conventional forms of corrosion.
- The very presence of the microbes prevents corrosion inhibitors from reaching and passivating the metal surfaces.
- Corrosion reactions are accelerated by microbiological interactions.
- Microbial by-products can be directly damaging to the metal.
The most common form of bacteria involved in MIC are Sulphate Reducing Bacteria (SRB’s).
Having a large microbiological population in a cooling water systems is less than desirable, and it is therefore imperative that these numbers are monitored regularly so their impact on the operating system can be minimised. As microbe numbers can proliferate at an exponential rate the more regular their numbers can be monitored the better.
Korrosion
Different alloys and non-metallic materials and their corrosion resistance taken from BG50*
| Material | Where used | Corrosion Resistance | Other Issues |
|---|---|---|---|
| Aluminium | some boiler heat exchangers and radiators | Good overall corrosion resistance in oxygenated waters of neutral or slightly alkaline pH. Should not be exposed | Exposure to high pH causes rapid loss of metal and formation or aluminium hydroxide sludge. |
| Copper & Copper Alloys | Copper tube, brass valves and fittings | Good overall corrosion resistance of neutral or moderatly alkaline pH. In aerated water, copper is subject to attack from erosion corrosion, flux residues and under deposit corrosion. | Copper ions entering the water can result in pitting corrosion of steel. Brass can be subject to stress corrosion cracking due to external contamination. |
Mild Steel & Cast Iron | Steel pipe, boiler heat exchangers, circulating pumps | Low levels of dissolved oxygen result in uniform corrosion and the production of magnetite sludge. High levels of dissolved oxygen result in pitting attack under tubercles. | Formation of insoluble iron oxides as suspended solids increase wear in pumps and the risk of under-deposit corrosion in low flow areas where sedimentation occurs. |
| Galvanised Steel | Some piping systems | Internally galvanised pipes and fittings should not be used in heating systems | Formation of zinc hydroxide as suspended solids. |
| Stainless Steel (SS) | Plate heat exchangers, pump castings, minor parts Occasionally pipework | Very good resistance to general corrosion but may be susceptible to pitting, crevice corrosion and stress corrosion cracking at high chloride concentrations. | none |
| Plastic | Plastic pipe including underfloor heating Minor parts | Resistance to corrosion but may be subject to physical degradation e.g. by sunlight. | Oxygen permeation through plastic pipe. Pressure resistance decreases with temperature. |
| Rubber | Flexible hose liners (EPDM), O-rings and seals | Resistant to corrosion but may be subject to gradual chemical and physical degradation leading to loss of flexibility and cracking. | Amenable to the formation of biofilm. |
*(BG50/2021 "Water Treatment for Closed Heating and Cooling Systems" – 2nd edition, Dr. P. Simpson)
Scale
Typically, hardness scale is a precipitation of calcium and magnesium compounds (e.g., calcium carbonate, magnesium silicate). It can reduce system life, increase energy consumption, maintenance, and operational costs by forming a tough deposit in HVAC cooling systems and process water systems. As water temperature increases, calcium carbonate becomes less soluble, therefore in cooling water systems, scale typically deposits on hottest surfaces, such as heat transfer sites. As a result of this highly insulating deposit, the system has a reduced capacity to transfer heat. Additionally, as scale takes up space in the pipework, it can also lead to reduced flowrates.
It not only takes more effort to move energy through this scale, but the corrosion inhibitors can no longer adsorb to the system metallurgy, potentially leading to under-deposit corrosion.
The quantity and likelihood of scale deposition is affected by a number of parameters including high calcium, magnesium, alkalinity and pH and therefore it is essential to monitor those parameters.
In most instances, a measure of the water systems Langelier Saturation Index (LSI) will be performed on the Make Up water and theoretical 'cycles' of this water to see how many cycles of concentration you can safely run to, based on the expected performance of the scale/corrosion inhibitor in use.